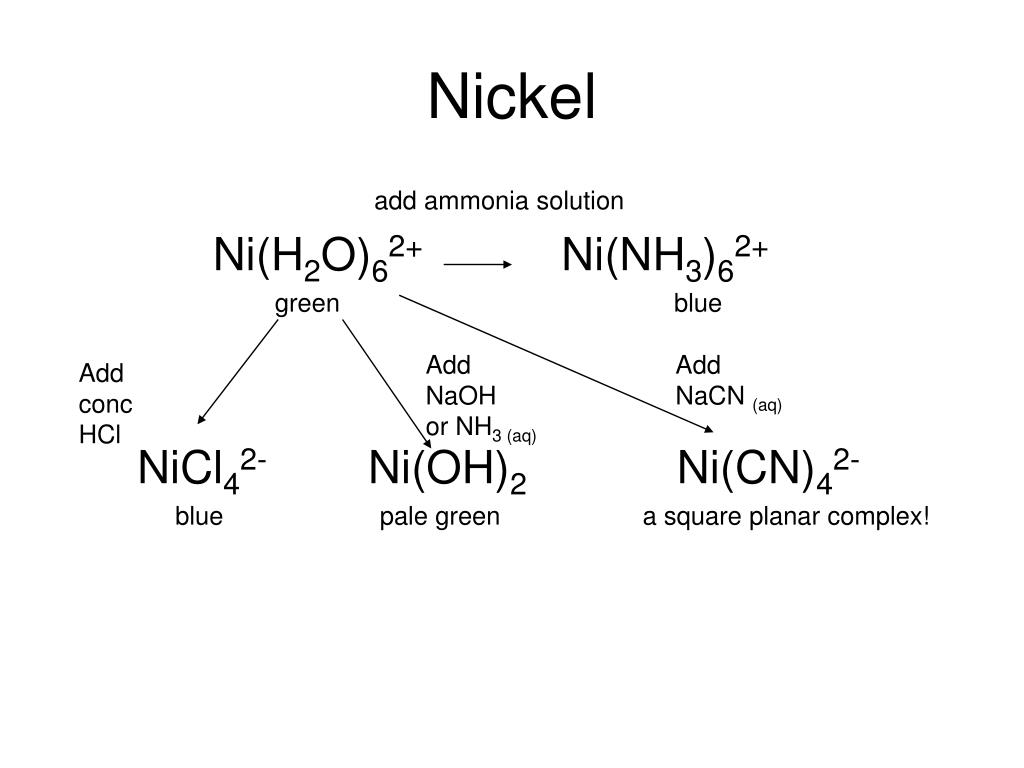
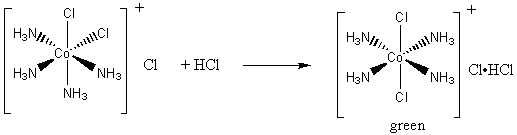E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder
![On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into](https://d1hhj0t1vdqi7c.cloudfront.net/v1/RHg2ME42M0hqRlU=/hq/)
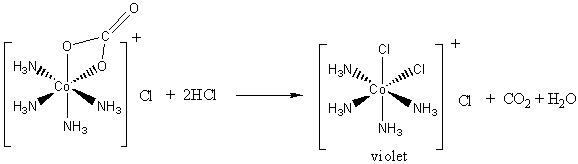
On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into
![On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into](https://i.ytimg.com/vi/Dx60N63HjFU/maxresdefault.jpg)
On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into
![Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni (OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni (OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com](https://homework.study.com/cimages/multimages/16/1.1.17267916333899178067.png)
Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni (OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com

OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...
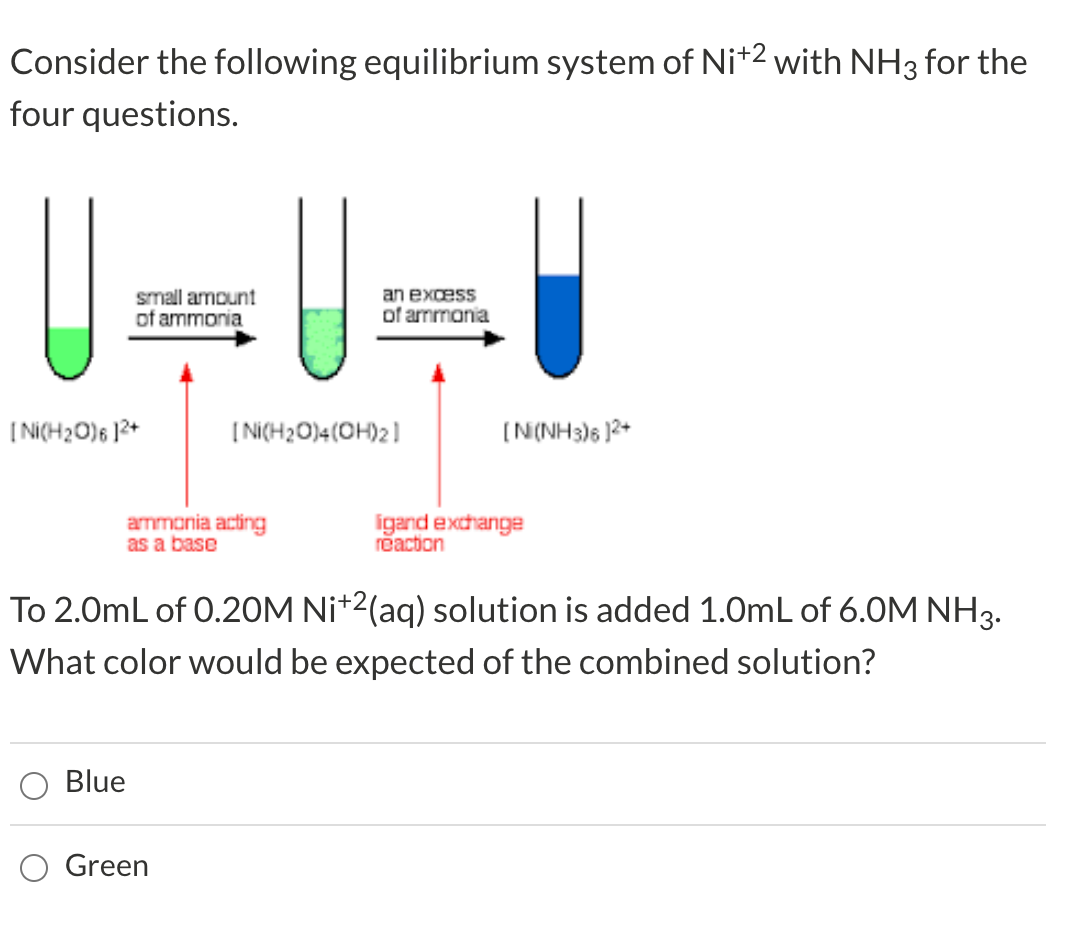
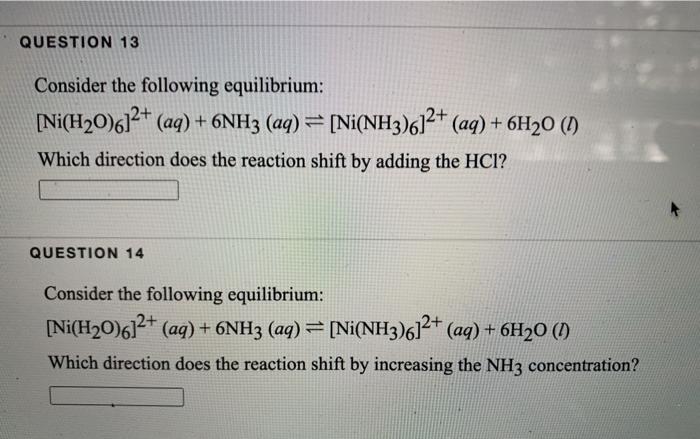
![SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale](https://cdn.numerade.com/ask_images/789c0aaf514c4932b19dafca225933f9.jpg)
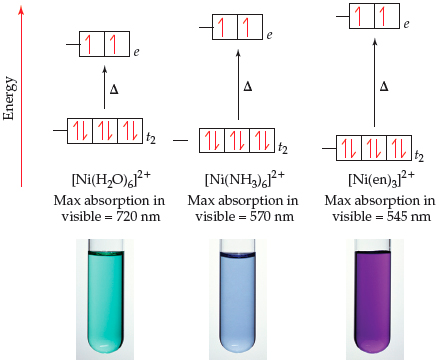
SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale

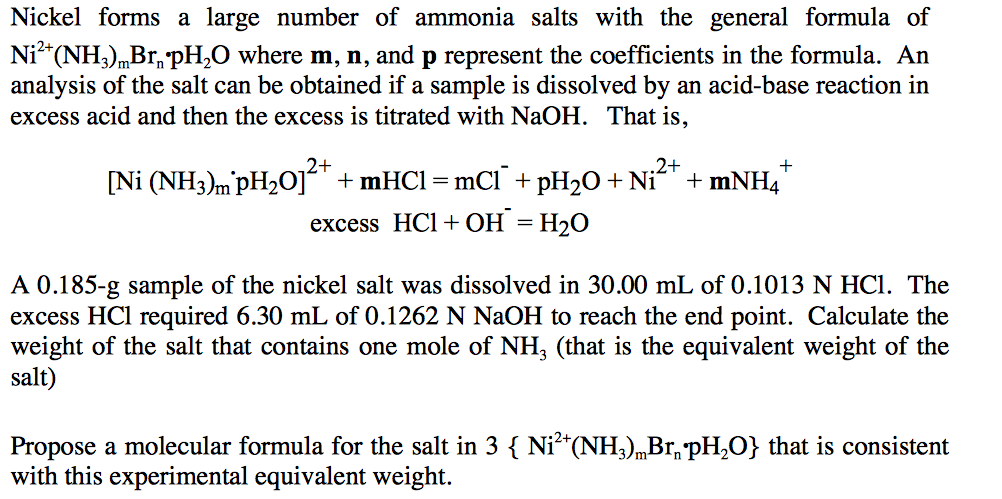
Experiment 5 –Synthesis and Stoichiometric Analysis of Hexaamminenickel (II) Chloride | SIC1002 - Inorganic Chemistry I - UM | Thinkswap
![SOLVED: 1. Write the chemical reactions (balanced) for the following reactants. NiCl2 and HCl to produce [NiCl6]4- [NiCl6]4- and H2O to produce [ Ni(H2O)6]2+ [Ni(H2O)6]2+ and Na2EDTA to produce [Ni(EDTA)] [Ni(H2O)6]2+ and NH4OH SOLVED: 1. Write the chemical reactions (balanced) for the following reactants. NiCl2 and HCl to produce [NiCl6]4- [NiCl6]4- and H2O to produce [ Ni(H2O)6]2+ [Ni(H2O)6]2+ and Na2EDTA to produce [Ni(EDTA)] [Ni(H2O)6]2+ and NH4OH](https://cdn.numerade.com/ask_previews/9fa67892-2e91-49e4-9a3b-403dad230868_large.jpg)
SOLVED: 1. Write the chemical reactions (balanced) for the following reactants. NiCl2 and HCl to produce [NiCl6]4- [NiCl6]4- and H2O to produce [ Ni(H2O)6]2+ [Ni(H2O)6]2+ and Na2EDTA to produce [Ni(EDTA)] [Ni(H2O)6]2+ and NH4OH
2 - YouTube 15.86 | Calculate the equilibrium concentration of Ni2+ in a 1.0-M solution [Ni(NH3)6](NO3)2 - YouTube](https://i.ytimg.com/vi/lyCYImCEQoA/maxresdefault.jpg)
15.86 | Calculate the equilibrium concentration of Ni2+ in a 1.0-M solution [Ni(NH3)6](NO3)2 - YouTube











![CHEMSOLVE.NET: [Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic CHEMSOLVE.NET: [Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic](https://1.bp.blogspot.com/-te3wP7SSHNM/Xrd1Gz5SLTI/AAAAAAAADJ8/Y7tjYvS9wsU8-SpoSm0_tCt8LGz0TqQJQCLcBGAsYHQ/s1600/Ni%2528II%2529%2B-%2BCopy%2B-%2BCopy.png)